CONCLUSION
Despite the development of new agents and the widespread use of hematopoietic cell transplantation (HCT), the prognosis of patients (pts) with multiple myeloma and extramedullary involvement (EMM) is rather unfavorable. Until now, there are no generally accepted standards for diagnosis and treatment of EMM.
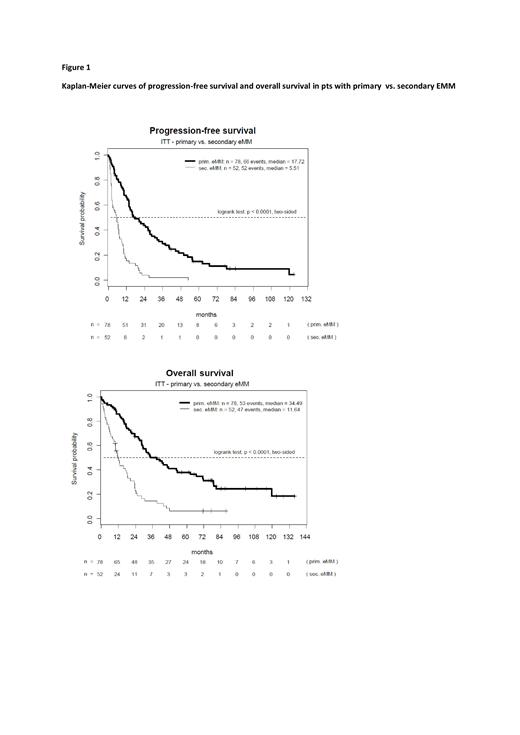
We performed a retrospective analysis of 130 pts with EMM treated at Klinikum Chemnitz between 2007 and 2022. Various demographic and clinical factors were recorded in a database and evaluated for their impact on response and survival. The data cut point was July 2022, and for follow-up a minimum of 3 months was required. One hundred and thirty pts (89 men, 41 women) with EMM at a median age of 68 years (range, 37-87 years) were included. In 78 pts extramedullary involvement was detected at the time of initial diagnosis (primary EMM), and in the remaining 52 pts a secondary EMM was diagnosed at the time of relapse (n=38) or refractory disease (n=14). In approximately two-thirds of pts EMM manifested exclusively in the paraskeleton (n=84), while roughly one-third of pts showed an extramedullary organ involvement (n=25), or both (n=21). Cytogenetic data were available in 125 pts, with 39 pts classified as high-risk EMM (including del17p in 11 pts). ISS could be evaluated in only 56 pts, of whom 22 pts had ISS III. Elevated serum LDH levels were observed in almost half of the pts (n=63) at the time of EMM diagnosis. Treatment regimens included mainly proteasome inhibitors (n=106), immunomodulatory drugs (n=74) and CD38 antibodies (n=42). Fifty-five pts (42.3%) received autologous (n=44), allogeneic (n=8) or autologous-allogeneic HCT (n=3).
Serologic response to treatment (at least PR) according to IMWG criteria was 63.8% (83/130) and imaging response was 56.9% (74/130). With a median follow-up of 84.2 months, median progression-free survival was 10.3 months and median overall survival was 23.8 months. In univariate analysis for PFS and OS, we found significantly worse outcomes in patients with secondary EMM (vs. primary EMM), EMM with organ manifestation (vs. paraskeletal EMM only), ISS III (vs. ISS I +II), and elevated serum LDH (vs. not elevated). Furthermore, pts with del17p had significantly shorter PFS, while detection of ampl1q32 (n=14) was associated with significantly worse OS. For other tested factors [lambda light chain involvement, t(14;16), dupl1q21 or tripl1q21, EMM diagnosis 2007-2013 vs. 2014-2022] we found no negative effects on PFS or OS. Multivariate Cox regression analysis confirmed secondary EMM as an independent negative factor for PFS (HR 3.62, p<0.0001) and OS (HR 2.95, p<0.0001), whereas detection of del17p was only associated with unfavorable PFS (HR 2.02, p=0.03).
Our data show that the prognosis of pts with EMM remains poor. Despite the introduction of many innovative agents, outcomes have not improved significantly since 2013. There is an urgent need for diagnostic/therapeutic guidelines of EMM as well as risk-adapted therapeutic strategies (using modern immunotherapies and cell therapies).
Disclosures
Zolnowski:Janssen-Cilag: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Roche: Honoraria; Ipsen: Honoraria; Novartis: Honoraria. Fricke:Kite/Gilead Sciences: Consultancy, Honoraria; Vertex Pharmaceuticals: Consultancy, Honoraria; MSGO: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V.: Patents & Royalties, Research Funding; Novartis: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria. Hänel:Bayer Vital: Consultancy; Sanofi: Consultancy; Gilead: Consultancy; Jazz: Consultancy; GSK: Consultancy; Takeda: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Honoraria; Sobi: Honoraria; Roche: Consultancy; Celgene: Consultancy.